Introduction: Myelofibrosis (MF) is a chronic myeloproliferative neoplasm characterized by the key hallmarks of constitutional symptoms, splenomegaly, and cytopenias such as anemia and thrombocytopenia. While Janus kinase (JAK) inhibitors have become a mainstay of MF treatment, improving symptom and spleen burden in many patients (pts), some approved JAK inhibitors such as ruxolitinib (RUX) may worsen or fail to adequately address anemia and thrombocytopenia. Dose reduction may mitigate the myelosuppressive effects of RUX, but this strategy and resultant effects on real-world clinical outcomes are not well characterized. Here we describe real-world baseline characteristics and posttreatment hematologic outcomes in a US pt population with MF treated with RUX, including those with red blood cell (RBC) transfusion burden at baseline.
Methods: This was a retrospective analysis of adult pts in the US Flatiron Health electronic health record-derived deidentified database with evidence of MF ( International Classification of Diseases, Ninth Revision or Tenth Revision diagnosis) on or after January 1, 2013, and ≥1 administration of an antineoplastic agent; the data cutoff was March 1, 2023. Pts were required to have ≥1 order or administration of RUX after MF diagnosis. Follow-up was from initiation of RUX (index date) to earliest of end of clinical activity, data availability, or death; only pts with index dates in April 2013 or later were included to allow for a 3-month baseline period.
Baseline characteristics were summarized at index date (or closest available visit ≤3 months prior) and included demographics, disease characteristics, hematologic measures, transfusion status, and prior treatment. Incidence of anemia, thrombocytopenia, and either anemia or thrombocytopenia (defined as a negative hematologic outcome), based on laboratory values (hemoglobin [Hb] levels <10 g/dL, platelet counts <150×10 9/L) or the presence of diagnostic codes, were evaluated in time-to-event analyses over the follow-up period. Subgroup analyses in pts who were transfusion dependent (TD; ≥4 RBC units transfused in any 8-week period, or ≥1 Hb level <8 g/dL at any point, within the previous 12 weeks) at baseline were also performed. Transfusion status at specific time points during follow-up was summarized.
Results: A total of 383 pts with MF treated with RUX in the real-world database met the criteria for analysis. The mean age was 72.0 years, 44.4% were female, and 72.4% were White. A total of 58.2% had primary MF; mean time from diagnosis to index was 8.7 months, and 22.2% had prior non-JAK inhibitor therapy before RUX initiation. Cytopenias were common at baseline; 45.7% had anemia and 24.5% had thrombocytopenia, based on laboratory assessment or diagnostic codes. Among pts with available Hb laboratory values, 13.4% had severe (<8 g/dL) and 38.5% had moderate (≥8 to <10 g/dL) anemia. A total of 20.9% were TD, with a mean 0.6 RBC units transfused per month.
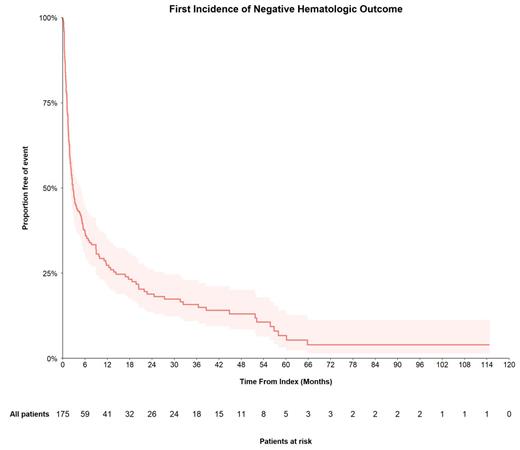
In time-to-event analyses, among the 175 pts who did not have either anemia or thrombocytopenia at baseline, 149 (85%) had an incident negative hematologic outcome (ie, anemia or thrombocytopenia) event during follow-up. The 6- and 12-month event rates were 63.5% and 72.7%; median time to event was 2.7 months (95% CI, 2.1-4.9 months) (Figure). In the respective subpopulations without anemia (n=208) or thrombocytopenia (n=289) at baseline, median time to anemia was 3.8 months (95% CI, 2.4-6.7 months), and median time to thrombocytopenia was 10.9 months (95% CI, 7.7-19.7 months); 12-month event rates were 66.8% and 51.8%.
At 3 months after index date, 41.3% of pts were TD. Among those who were TD at baseline, 88.8% remained TD after 3 months of RUX treatment, with a mean increase in transfusion intensity of 0.4 units per month. Among pts who were not TD at baseline, 24.4% became TD after 3 months of RUX treatment, with a mean increase in transfusion intensity of 0.6 units per month.
Conclusions: This retrospective analysis provides insights into hematologic outcomes under the current first-line treatment paradigm for MF in clinical practice. Many pts who initiated RUX were anemic and/or thrombocytopenic at baseline, and incidences of anemia and thrombocytopenia as well as RBC transfusion burden increased over time. These results highlight the continued need for treatments that address the underlying hematologic burden, including anemia and thrombocytopenia, of pts with MF.
Disclosures
Kuykendall:BMS: Consultancy, Research Funding; Blueprint: Consultancy, Research Funding, Speakers Bureau; Morphosys: Consultancy, Research Funding; Protagonist Therapeutics, Inc.: Consultancy, Research Funding; Prelude: Research Funding; CTI: Consultancy; AbbVie: Consultancy; Sierra Oncology: Research Funding; Imago: Consultancy; GSK: Consultancy; Incyte: Consultancy; Novartis: Consultancy. Liu:GSK: Current Employment. Zhang:GSK: Other: Employee of analysis group which received funding for this research by GSK . Simpson:GSK: Other: Employee of analysis group which received funding for this research by GSK . Phiri:GSK: Current Employment, Other: Stock Holder.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal